electronic configuration for iodine|Iodine Electron Configuration: Everything You Need to Know : Bacolod Iodine electron configuration notation. The electronic configuration notation of [I] is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4d10 5s2 5p5. The electronic configuration notation . Blocked? Try geometry.monster | The OFFICIAL home of Shell Shockers, the world’s best egg-based shooter! It’s like your favorite FPS battlefield game. with eggs.
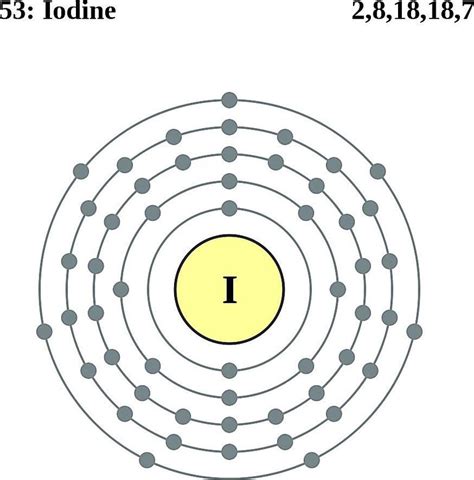
electronic configuration for iodine,Iodine Electron Configuration: Iodine is a chemical element that has a symbol I. The atomic number of Iodine is 53. It is the heaviest .Electron configuration for iodine. The history of Iodine. Periodic table history. Identifiers. List of unique identifiers for Iodine in various chemical registry databases. Iodine is a .
Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. .Iodine. Full electron configuration of iodine: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5. tellurium ← iodine → xenon. Iodine, complete electron configuration.
Iodine electron configuration notation. The electronic configuration notation of [I] is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4d10 5s2 5p5. The electronic configuration notation .Iodine Electron Configuration: Everything You Need to KnowElectronic configuration of the Iodine atom. Valence electrons. Orbital diagram. In order to write the I electron configuration we first need to know the number of electrons for the I atom (there are 53 electrons). When we write the .

Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration (or Noble gas configuration) as well as Full .
What is the electronic configuration of Iodine? The electronic configuration of Iodine is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁵. How many electrons are present . Electron Configuration and Oxidation States of Iodine. Electron configuration of Iodine is [Kr] 4d10 5s2 5p5. Possible oxidation states are +1,5,7/-1. Electron Configuration. The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers, electron configurations, and chemical .The Electron configuration of iodine is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s²4d¹⁰ 5p⁵. Iodine, also called iodine is defined as the chemical element that belongs to the periodic table. It is located in group 17, more precisely in the halogens, its atomic number is 53 and it is represented by the symbol I. .

The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer .
Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for Mg 2+ will be 1s²2s²2p⁶.
Excited state Iodine electron configuration. The first excited state electronic configuration of [I] is [Kr] 4d 10, 5s 2, 5p 4, and 5d 1, which is characterized by the transition of one electron from 5d to 6s orbital, creating 5d 8 and 6s 2 in the outermost shell.; The first excited state of Iodine How does the electronic configuration of Iodine explain its chemical properties? Iodine has 7 valence electrons, which makes it highly reactive and prone to forming chemical bonds with other elements in order to complete its octet. This reactivity allows Iodine to form a wide range of compounds with other elements, including metals .electronic configuration for iodine There are two ways to find the number of valence electrons in Iodine (I). The first is to use the Periodic Table to figure out how many electrons Iodine has .
In this video we will write the electron configuration for I- the Iodide ion. We’ll also look at why Iodine forms a 1- ion and how the electron configuration. The iodine orbital diagram is a graphical representation of the electron configuration of the iodine atom. This diagram shows how the electrons in the iodine atom are arranged in different orbitals. Orbital is the region of space around the nucleus of an atom where electrons are found. The elements of Group VIIA (new Group 17 - fluorine, chlorine, bromine, iodine, and astatine) are called the halogens (yellow column). The term “halogen” means “salt-former” because these elements will readily react with alkali metal and alkaline earth metals to form halide salts. . The electron configuration in the outer shell is .Protons and Neutrons in Iodine. Iodine is a chemical element with atomic number 53 which means there are 53 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.Abbreviated electronic configuration of Iodine. The ground state abbreviated electronic configuration of Neutral Iodine atom is [Kr] 4d10 5s2 5p5. The portion of Iodine configuration that is equivalent to the noble gas of the preceding period, is abbreviated as [Kr]. For atoms with many electrons, this notation can become lengthy and so an .
The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number .
Configuration. σ g 2 π u 3 π g 3 σ u 2. 7 . of the D state excited by the 1830 atomic line of iodine. The system further includes the diffuse emission bands in the region 2500 - 5000 with a . Schwarz, W.H.E., Inner electron excitation of iodine in the gaseous and solid phase, J. Chem. Phys., 1973, 58, 2230. Venkateswarlu, 1970 .electronic configuration for iodine Iodine Electron Configuration: Everything You Need to KnowNote that these electron configurations are given for neutral atoms in the gas phase, which are not the same as the electron configurations for the same atoms in chemical environments. In many cases, multiple configurations are within a small range of energies and the irregularities shown below do not necessarily have a clear relation to . The electron configuration of iodine is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 5. Now in the next step, start drawing the orbital diagram for iodine. Draw orbital diagram. Before drawing the orbital .Element Iodine (I), Group 17, Atomic Number 53, p-block, Mass 126.904. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs.
Noble Gas Configuration. The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\) (Table \(\PageIndex{1}\)). The first ten electrons of the sodium atom are the inner-shell electrons and the configuration of just those ten electrons is exactly the same as the configuration of the element neon \(\left( Z=10 \right)\).
electronic configuration for iodine|Iodine Electron Configuration: Everything You Need to Know
PH0 · Iodine, electron configuration
PH1 · Iodine – Electron Configuration and Oxidation States – I
PH2 · Iodine Electronic Configuration and Distribution in Shells
PH3 · Iodine Electron Configuration: Everything You Need to Know
PH4 · Iodine Electron Configuration (I) with Orbital Diagram
PH5 · Iodine (I)
PH6 · Iodine
PH7 · Electron configuration for Iodine (element 53). Orbital diagram
PH8 · Electron Configuration Chart of All Elements (Full Chart)
PH9 · Electron Configuration
PH10 · Complete Electron Configuration for Iodine (I, I– ion)
PH11 · A step